WATER SURVEYS
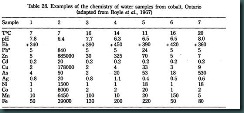
Even very ancient history books refer to mineral prospecting by qualitative testing of water from seeps, springs, streams, wells and bores. However, two basic problems have hindered quantitative analysis in water samples in modern geochemical prospecting. The first problem (is that the concentration levels of indicator elements in natural water samples very low (generally a few ppb), thereby posing both analytical difficulties and a serious risk contamination during processing of samples ( glass or plastic containers and suspended particles can either contribute elements to or subtract elements from the sample unless Suitable. precautions are taken). The second problem is that the chemistry of natural water is very sensitive to weather conditions and to its local environment. Consequently, geochemists have shown an understandable reluctance to use water samples in exploration. However, recent analytical advances have made it feasible to detect suitable indicators by using practical sample sizes of 100-500m1 of water (samples are generally concentrated by evaporation or solvent extraction prior to analysis), and refined interpretation techniques have reduced the difficulties of interpreting water chemistry. This has stimulated interest in stream and lake water sampling in glaciated terrains and groundwater sampling in arid terrains. Much of the attention centers on U, but other types of deposits can be sought A paper by Boyle et al. (1971) has reviewed the theory and practice of hydro geochemical methods, although with special reference to Canadian applications. It describes sound methods of sampling and interpretation and points out many of the pitfalls. Although the methods have received little attention in Canada, because of a widespread belief that sediment sampling is more effective, the authors predicted that there is scope for increasing use of hydro geochemical methods in both reconnaissance and detailed exploration. The Canadian Geological Survey has since embarked on large scale experiments with sampling of lake water and sediment. A general review of the applications of hydro geochemistry to the search for base metals has been published by Miller (1979) and brief summary comments on the use of water samples specifically in Australia have been made by Mann (1980). EXAMPLES Brundin & Nairis (1972) investigated the relative effectiveness of sediment sampling, organic matter sampling and water sampling in northern Sweden. Their results indicated that in that glacial terrain the best information was obtained by sampling organic matter in the streams. However, both water sampling and organic matter sampling were found to be equally as good as sediment sampling for regional prospecting for Mo and Zn. Both water samples and organic samples were found to be more suitable than stream sediments in regional prospecting for U. It must be noted that the Swedish investigation was prompted by disadvantages of stream sediment sampling in glacial terrains (mainly lack of suitable sediment over long distances in streams, and widely ranging contents of scavenger substances) so that it would be wrong to assume that under Australian climatic conditions, stream sediment sampling would necessarily be less effective than water or organic matter sampling. However, the findings of Brundin & Nairis may well be relevant to prospecting in southern New Zealand. They also bear testing in any region where stream sediment sampling is difficult; for example, in New Guinea the rugged topography and high rainfall cause so much flushing of streams that collection of even sand-sized sediment is difficult. Water samples would certainly be easier to collect, if it can be demonstrated that they are effective samples in that region.. Experiments have been conducted on the use of stream water samples in non-glaciated terrain but in general there is less justification, and stream sediment sampling remains more economical and results can be interpreted more confidently. One notable exception to this generalization is the analysis of F in stream water in search of fluorite of Sn deposits. Field determinations of F present at low concentrations in stream water can be made in the field using a fluoride ion sensitive electrode which is similar in size and operation to a pH meter. Despite the fragile nature of the electrode, useful results have been obtained under fully operational conditions in rugged high rainfall terrain in Australia. In arid regions where there is inadequate drainage for sediment or stream water survey sand unsuitable overburden for soil sampling the choice for geochemical sampling shrinks to bedrock or groundwater. Bedrock dispersion patterns are generally small and collection of samples may necessitate expensive drilling. On the other hand, water samples collected from bores sunk for domestic or stock use are available cheaply and may delineate large hydromorphic anomalies which subsequently can be examined in detail by bedrock sampling or by water sampling of prospecting boreholes sunk on a grid pattern. The paucity of literature on groundwater sampling conveys a fake impression of inactivity. Numerous surveys have been conducted for U, V, Cu, Pb, Zn and other elements but without conspicuous success. In many cases it is not clear whether failure can be attributed to lack of mineralization or failure to detect mineralization. More care is needed to interpret anomalies in water than in rock, soil or sediment because anomalies are generally of low contrast and need to be interpreted by refined procedures, such as regression analyses, which take into account important factors such as pH, Eh, temperature, associated soluble species, etc, which compete to control the abundance of any given indicator element. Trost & Trautwein (1975) investigated Eh, pH, conductivity, Mo, SO. and other trace elements in groundwater samples collected from wells in a. porphyry Cu area in Arizona. Mo was found to increase in the vicinity of mineralization and a high ratio Mo/conductivity was suggested as a useful indicator of mineralization. Previous studies by Huff (1970) had also indicated Mo anomalies in groundwater near porphyry Cu but Cu itself was found to be useless as an indicator. One documented example of water sampling which led to discovery of additional mineralization is a spring water sampling program in the southwest Wisconsin area of the Mississippi Valley Pb-Zn district (De Geoffroy et at. 1968; De Geoffroy & Wu, 1970). An area of about 2250 km2 was investigated using 7210 water samples in which the Zn content ranged between 0.03 and 3.50 ppm. The method of interpretation was based on examination of regional variation trends and residual components. It outlined 122 target areas, 42 of which are known to contain Zn deposits. By l970theflrst5of thereniaining80targets, not associated with known deposits, had been tested by drilling (DeGeoffroy & Wu, 1970). Zn mineralization of commercial grade was intersected in several drill holes in each of the 5 targets. Two recent contributions from the U.S.S.R. also indicate activity in groundwater sampling (Kraynov, 1971; Naumov et at. 1972). Both papers highlight the need for careful interpretation of “anomalies’ The paper by Naumov et at. (1972) is particularly important in providing a. critical discussion of the behavior of Cu, Bi, Ag, Pb, Zn and SO in groundwater surrounding deposits in a desert area. Bi and Zn proved to be far superior to the other elements in producing large and discernible dispersion patterns,. Sulphate abundance in either surface or groundwater's has been proposed frequently as a potential indication of oxidizing sulphide deposits. A method proposed by Dall’Aglio & Tonani (1973) for discriminating between SOt produced by oxidizing suiphides and that attributable marine sources (e.g., gypsum) may prove quite important. Their method consists of plotting or regressing SO versus Ca or Cl to distinguish stream water samples containing more SOt than can be accounted for by derivation from sedimentary sulphate sources. Similar methods of interpretation of SOt content in groundwater collected from sulphide mineralized, but gypsies, arid terrain in Australia is claimed to show promising results. It is apparent that there has been interest and activity in Australia in using water sampling for U prospecting. Some comments and results are given by Mann (1980) and Deutscher et at. (1979). The most conventional procedure in U prospecting is radiometric surveying. Whilst this is a fast, simple procedure, it is limited in its application by the fact that gamma radiation can penetrate only a thin blanket of overburden or water. One solution to this problem is to prospect radio metrically for Rn gas. However, gaseous Rn diffusion is prevented by regions of groundwater saturation (Andrews & Wood, 1972). Therefore, analysis of groundwater (or stream water fed by groundwater) for U or Rn has the potential to reveal more deeply concealed U mineralization than can be detected by surface radio metric surveying. Bowie et at. (1971), Andrews & Wood (1972), Brundin & Nairis (1973) and Michie et al. (1973) all regard analysis of stream waters as a valid prospecting medium for U mineralization. Wenrich-Verbeek (1977) published a very informative study of the behavior of U and coexisting elements in surface waters and associated sediments with various sampling techniques used for U exploration. She includes the following important recommendations: 1. water samples should be filtered at the time of collection and acidified. 2. conductivity measurements should be made and used to normalize U determinations for evaporation and dilution effects related to steam discharge rates. 3. sediments intended for U analysis should be sieved to yield a very fine fraction (less than170 mesh). 4. U anomalies in water are not necessarily reflected in stream sediments, and vice versa, so sampling of both water and sediment is recommended. Using similar reasoning to that applied to Rn, Clarke & Kugler (1973) claimed that near.. surface sampling of natural waters followed by mass spectrometric analysis for He isotopes also may be a valid prospecting method for U and Th deposits. Their recommendation was. based on tests of water from drill holes near known U deposits; total He enriched near mineralization. It is now feasible to perform the analyses in the field at threat of about 100 samples per day using a truck mounted spectrometer (Reirner et at. 1979),,L Most of the known areas of unmineralization in Australia occur in arid or semi-arid regions and, therefore, stream water sampling may be of limited benefit. However, because of their lack of surface water these arid regions often contain numerous deep bores which: should be of prime interest for water samples in regional prospecting. It has been realized, presumably retrospectively, that several of the U prospects contain water bores with anomalously high U contents. An alternative to direct collection of water for U analysis is the use of a collector device, such as an ion exchange resin. The field use of a resin has the following advantages: Consequently, Parslow & Dwairi (1977) designed and tested resin packages suitable for field use. The packages are resin-filled tea bags and values of as little as 0.1 ppb U in water can be detected with care. Some groundwater sampling for Cu (Rattigan et al. 1977) has been conducted by Pacminex on the Stuart Shelf using a cylinder and ball valve sampling tool lowered down bore holes but confident interpretation of the results was not achieved. Most of the data related to Cu and Zn but some samples were analyzed also for Pb and U. Cu contents as high as 580 ppb were encountered in formation waters in unmineralized terrain in contrast to values as low as 98 ppb near the Cattle Grid Cu deposit. A variation on the theme of water sampling which is of no practical interest in Australia (if anywhere) is provided by a paper on snow geochemical sampling (Jonasson & Allan, 1973). They demonstrated contents of Cu as high as 161 ppm and Zn up to 53 ppm in snow samples overlying mineralization. The evidence suggests that these metals have diffused into the snow ionically via capillary water. Whereas this observation is of little practical concern, it is surprising to note that extensive hydro geochemical dispersion continues even under apparently frozen conditions. SAMPLING METHODS AND GENERAL COMMENTS Some points to be taken into consideration in using water samples are as follows: For example, in sampling wells or boreholes care must be taken to flush pumps,pipes, casings, etc. In all cases the sample containers should be rinsed thoroughly with dilute acid then metal-free water; they should be finally rinsed at least three times at the sample collection point, using the water intended for sampling. Despite its apparent homogeneity to the eye, water is just as prone to layering and incomplete mixing as any other geological material. 5. the temperature, pH, Eli, degree of aeration, rate of flow, amount of suspended matter, etc. should be noted at each sample point. 6. any samples which are to be compared must be collected in a short time interval to minimize variation in response to climatic factors. 7. it is necessary to filter the water through a O.45m filter during collection and possibly to acidify it prior to storage. 8. there is a finite storage life for many indicator elements in bottled samples so analyses should be made promptly. 9. the quantity required is between 500 mI and 2 liters of water acidified with 1-2 mis of metal-free HC1 — in order to permit gravimetric analysis of SO and atomic absorption analysis of the metals after solvent extraction. Samples of this size are bulky and the cost of analysis is about three times as great as that for sediment or soil samples. Some analysts are prepared to work with less and specific analyses may require no more than a few ml; for example, analysis of U in water by neutron activation requires only 1-5m1 of sample (Bowie et al. 1971). 10. an ion exchange resin may be substituted for an actual water sample in some circumstances. A guide to the type of abundances encountered is given in Table 28 which is extracted from information published by Boyle etaL (1967) in connection with orientation experiments in prospecting for Ag veins (which include Ni-Co arsenide's, and Pb, Zn, Cu and Bi minerals) in the Cobalt area of Ontario. Table 28. Examples of the chemistry of water samples from cobalt, Ontario (adapted from Boyle et al., 1967) *All elements are expressed in parts per billion (ppb). 1 Moderately flowing clear water from drill hole in Ag mine. 2 Groundwater from fault in Ag mine. 3 Clear well water from diabase. 4 Clear well water from diabase, H2S smell. 5 Clear water from old Ag mine shaft in Keewatin pillow lava. 6 Clear spring water from Keewatin pillow lava. 7 Rapidly flowing clear surface water on diabase. A more generalized table of the most frequently encountered and maximum contents of 26 indicator elements in supergene waters has been presented by Shvartsev et al. (1975) and reproduced by Miller (1979). Finally, general impressions are that water sampling has not been widely applied but has produced useful results in some cases. The most readily useful indicator elements for appropriate mineral deposits appear to be U, V, Rn, He, Mo, Zn, Bi, F and SO. Results obtained using Cu and Pb, two indicators commonly used in other forms of geochemical sampling, seem to be difficult to interpret. Certainly the results of water surveys are likely to demand refined interpretation methods, since many factors interplay to modify the abundance of an indicator element after it enters the water system. With care a wider range of indicator elements may prove useful in the future — including Ag, As, Be, Co, Ni, Sn and W REFERENCES ANDREWS, J.N. & WOOD, D.F., 1972. Mechanism of radon release in rock matrices and entry into groundwater's. Trans. Inst. Mm. Metal!., 81, B 206-209. BOWIE, S.H.U., OSTLE, D. & BALL, T.K., 1971. Geochemical methods in the detection of hidden uranium deposits. C.LM Spec. VoL 11, -103-111. BOYLE, R.W., DASS, A.S., CHURCH, D., MIHAILOV, G., DURHAM, C., LYNCH, J. & DYCK, W, 1967. Research in geochemical prospecting methods for native silver deposits, Cobalt area, Ontario, 1966. Ceo!. Surv. Canada Paper 67-35.



No comments:
New comments are not allowed.